Autocrine
( autocrine growth factor ; sometimes abbreviated: AGF ) A term referring to a mechanism of growth
control, called also autostimulatory growth control , in which a cell secretes a soluble
factor (see also: Conditioned medium ) that binds to its receptor, which is
expressed also by the factor-producing cell. This effectively creates an
autogenous loop in which a product (for example, Cytokines ) acts back on the cells that produce it.
|
|
|
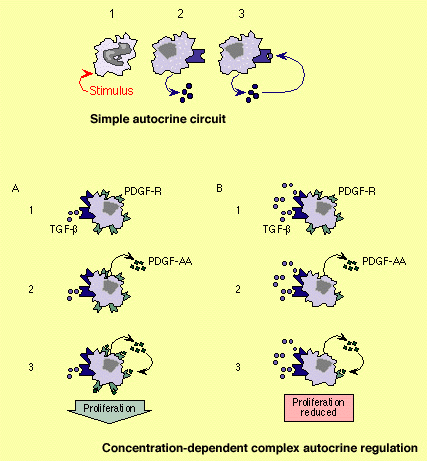
Simple
and complex autocrine growth control loops. Upper panel: The
stimulation of cells (1) induces the expression and secretion of a mediator
(2) and its membrane-bound receptor. By interacting with its receptor (3) the
mediator can elicit further responses in the cell by which it is produced. Lower panel: Concentration-dependent
complex control of an autocrine loop. A) At low concentrations
binding of TGF to its membrane receptor (1) induces the
production of PDGF-AA (2). PDGF-AA then binds to its own receptor which is also
expressed by the cell and thus can stimulate cell proliferation. B ) High concentrations of TGF (1) down-regulate expression of receptors for
PDGF-AA (2). Biological responses elicited
by PDGF-AA are thus diminished due to a
paucity of PDGF-AA receptors. The effect is reduced
cell proliferation. Concentration-dependent differential actions involving an
interplay of several factors and down-regulation of receptors have been
observed also for a number of other cytokines. |
Autocrine loops effected through the interaction of growth factors with their corresponding receptors can take
simple forms involving only a single growth factor or rather complex forms
involving two or more growth factors .
|
|
|
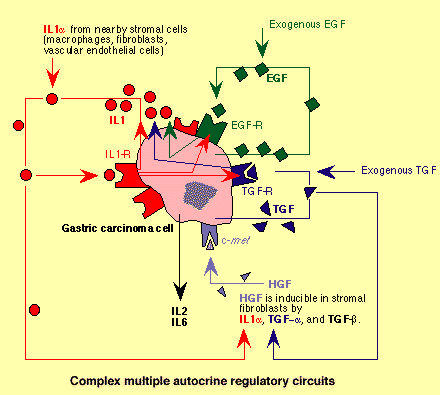
Example
of complex multiple autocrine growth control loops in gastric carcinoma
cells. Gastric carcinoma cells
secrete large amounts of IL1-alpha and express significant amounts of the IL1 receptor. IL1-alpha can enhance expression of itself and is
provided also by nearby stromal cells such as macrophages, fibroblasts, and
vascular endothelial cells. In addition, production of IL1-alpha by gastric carcinoma cells is induced also by
EGF and TGF-alpha . Autocrine growth of gastric carcinoma cells
mediated by an interplay between IL1 and its receptor is demonstrated by the fact
that antibodies against IL1-alpha and also IL1 receptor antagonist (IL1ra ) significantly suppress cell growth. Gastric carcinoma cell
lines also produce EGF and TGF-alpha and their receptors. IL1-alpha can induce expression of EGF and TGF-alpha receptors. EGF and TGF-alpha can induce expression of IL1-alpha . EGF and TGF-alpha can induce the synthesis of their own
receptors and also of stromelysin and collagenase . This sets in motion a cascade of events
leading to extracellular matrix degradation, which is convenient
for tumor progression and invasion. HGF (hepatocyte growth factor ) can be produced by stromal
fibroblasts and its synthesis by these cells can be enhanced by TGF-alpha , TGF-beta , and IL1-alpha . HGF can also stimulate growth of gastric
carcinoma cells which express the HGF receptor met . In addition, gastric carcinoma cells can
also secrete IL2 and IL6. |
A study designed by Laufenberger et al to measure autocrine signaling through
the EGF receptor by TGF-alpha has revealed that autocrine signaling can be
blocked more effectively with anti-receptor antibodies than with anti-ligand
antibodies. This result seems to suggest that local restriction of
autocrine-based signals may provide cells with information on their local
microenvironment.
The uncontrolled synthesis
of such autocrine growth factors by cells that also express the corresponding
receptors is a mechanisms allowing, for example, tumor cells to regulate their
own growth and to become independent of exogenous growth control mechanisms
(see also: Factor-dependent cell lines ). In some cases an activated Oncogene is responsible for the constitutive synthesis
of aberrant proteins that take over the role of authentic growth factors . The regulated expression of growth factor
receptors observed frequently appears to be a safeguard against a catastrophic
spread of cell proliferation by a growth factor stimulus. Again, the
uncontrolled expression of receptor genes subverts this mechanism of orderly
growth.
The existence of such
autocrine loop in tumor cells has been demonstrated by in vitro experiments
showing that factor-specific neutralizing antibodies are capable of inhibiting
further proliferation of the factor-producing cells. The immunization of mice
with an autocrine growth factor effectively blocks the in vivo
growth of the tumor cells expressing and secreting this growth factor (see: hst ).
Examples for the endogenous
production of tumor-associated growth factors are, among many others, IL3 , EGF , bFGF , IGF and PDGF . In some instances autocrine loops have been
shown to be very complicated mechanisms involving more than a single factor and
its receptor gene.
The establishment of
autocrine growth control loops is not restricted to regulatory peptide factors.
It has been observed, for example, that Epstein-Barr virus-transformed
B-lymphocytes secrete protein factors but that the main mediator allowing
autocrine growth is lactate. The neurotransmitter serotonin appears to be an autocrine growth factor for small cell lung carcinomas (see
also: Bombesin ).
The autocrine growth
control model is the basis for a number of therapeutical concepts that, at
least in principle, should allow direct interference with the growth control
mechanisms of tumor cells.
A reduction in the tissue
and plasma concentrations of growth factors can be achieved by the administration of
antibodies directed against the growth factor. At least experimentally this has
been shown to inhibit the growth of Factor-dependent cell lines derived from tumor cells and also of
human tumor cells transplanted into nude mice (see also: Immunodeficient mice ).
The reverse, i. e. the
provision of additional growth factors for the purpose of inhibiting tumor cell
growth, is based on the observation that many growth factors induce the down-modulation of receptor
expression (see also: Receptor transmodulation ). It may be envisaged that
increased doses of growth factors may induce resting tumor cells to enter the Cell cycle and hence to become vulnerable to chemotherapy.
A similar concept is used, for example, in the therapy of mammary carcinomas
which may involve low-dose treatment with estrogens before chemotherapy.
Another way of blocking
tumor cell growth might be a blockade of the growth factor receptors by
specific monoclonal antibodies. Antibodies directed against, and blocking, the EGF receptor have been shown to lead to tumor
regression of transplanted epithelial carcinomas in the nude mouse model (see also: Immunodeficient mice ).
Yet another possibility of
exploiting the autocrine growth control concept might be Antisense RNA that can be used to interfere with the
expression of growth factors and growth factor receptor genes at
the level of transcription (see also: Gene expression ).
For other mechanisms of
growth control see also: intracrine , juxtacrine , paracrine , retrocrine .
For other entries
pertaining to cell lines and cell culture see also: Cells MiniCOPE Dictionary .
Status: date of last
revision: 07/01/1999
References: Avis IL et
al Preclinical
evaluation of an anti-autocrine growth factor monoclonal antibody for treatment
of patients with small-cell lung cancer Journal of the National Cancer Institute 83: 1470-1476 (1991); Bajzer Z and
Yuk-Pavlovic S Quantitative aspects of autocrine regulation in tumors. Critical
Review of Oncogenesis 2: 53-73 (1990); Battegay EJ et al TGF-beta
induces bimodal proliferation of connective tissue cells via a complex control
of an autocrine PDGF loop. Cell 63: 515-524 (1990); Becker D et al Proliferation
of human malignant melanomas is inhibited by antisense oligodeoxynucleotides
targeted against basic fibroblast growth factor EMBO
Journal 8:
3685-3691 (1989); Dunn AR and Wilks AF Contributions of autocrine and
non-autocrine mechanisms to tumorigenicity in a murine model for leukemia. Ciba
Found. Symp. 148: 145-155 (1990); Forsten KE and Lauffenburger DA Autocrine
ligand binding to cell receptors. Mathematical analysis of competition by
solution "decoys". Biophys. Journal of 61: 518-529 (1992); Forsten
KE et al Interrupting autocrine ligand-receptor binding: comparison between
receptor blockers and ligand decoys. Biophys. Journal of 63: 857-861 (1992); Garbers
DL Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine
ligands. Cell 71: 1-4 (1992); Gordon J and Cairns JA Autocrine
regulation of normal and malignant B lymphocytes. Advances in Cancer Research
56: 313-334 (1991); Heldin CH and Westermark B Autocrine stimulation of
growth of normal and transformed cells. In: Habenicht A (edt) Growth factors,
differentiation factors, and cytokines, pp. 267-278, Springer, Berlin 1990; Jasmin
C et al Autocrine growth of leukemic cells. Leukocyte Research 14: 689-693
(1990); Lang RA and Burgess AW Autocrine growth factors and tumorigenic
transformation Immunology Today 11: 244-249 (1990); Lauffenburger
DA et al Real-time quantitative measurement of autocrine ligand binding
indicates that autocrine loops are spatially localized. Proceedings of the
National Academy of Science (USA) 195(26): 15368-15373 (1998); Leof EB et al
Induction of c-sis mRNA and activity similar to platelet-derived growth factor
by transforming growth factor-beta: a proposed model for indirect mitogenesis
involving autocrine activity. Proceedings of the National Academy of Science
(USA) 83: 2453-2457 (1986); Mulshine JL et al Autocrine growth factors
as therapeutic targets in lung cancer. Chest (Suppl.) 96: 31s-34s (1989); Nicolson
GL Paracrine/autocrine growth mechanisms in tumor metastasis. Oncol. Res.
4: 389-399 (1992); Nicolson GL Cancer progression and growth:
relationship of paracrine and autocrine growth mechanisms to organ preference
of metastasis. Experimental Cell Research 204: 171-180 (1993); Pike SE et al
The role of lactic acid in autocrine B cell growth stimulation. Proceedings
of the National Academy of Science (USA) 88: 11081-11085 (1991); Rodan GA et
al Autocrine/paracrine regulation of osteoblast growth and differentiation
Lab. Invest. 64: 593-595 (1991); Schüller HM Receptor-mediated mitogenic
signals and lung cancer. Cancer Cells 3: 496-503 (1991); Schwab G et al Characterization
of an interleukin-6-mediated autocrine growth loop in the human multiple
myeloma cell line, U266. Blood 77: 587-593 (1991); Sporn MB and Roberts AB Autocrine
growth factors and cancer Nature (London) 313: 745-747 (1985); Sporn MB and
Roberts AB Autocrine secretion: 10 years later. Ann. Intern. Med. 117:
408-414 (1992); Sporn MB and Todaro GJ Autocrine secretion and malignant
transformation of cells. New England Journal of Medicine 3093: 878-880 (1980); Trepel
JB et al A novel bombesin receptor antagonist inhibits autocrine signals in
a small cell lung carcinoma cell line. Biochemical and Biophysical Research
Communications 156: 1383-1389 (1988); Yayon A and Klagsbrun M Autocrine
regulation of cell growth and transformation by basic fibroblast growth factor.
Cancer Metastasis Rev. 9: 191-202 (1990); Walsh JH et al Autocrine
growth factors and solid tumor malignancy. West. Journal of Med. 155: 152-163
(1991)